Defects in brickwork
Contents |
[edit] Introduction
Defects can arise in new brickwork because of poor design or specification, the use of sub-standard materials and poor standards of workmanship.
[edit] Frost attack/damage
Frost attack/damage is a common problem that usually occurs in older bricks, and those that were underburnt during the firing process. In newer construction, failure through frost attack tends to be confined to areas of severe exposure, or where the frost resistance of the brick was incorrectly specified.
The ability of brick to resist frost attack is determined by their pore structure (in particular the percentage of fine pores in the brick). Frost attack occurs through a combination of excessively wet brickwork and freezing temperatures.
When water turns to ice, there is a 9% increase in its volume. This expansion can produce stress within the brick, which causes spalling, with the brick face flaking off and/or crumbling. Mortar is also subject to frost attack. In a deteriorating state both elements more readily absorb water which in turn increases the rate of frost damage.
Although the risk of frost attack is increased where the saturated brick is subjected to particularly low temperatures, it is the rapidity of the freeze-thaw cycle that causes the damage. As the process is progressive, frost attack can lead to total disintegration of the brick.
The actual level of moisture within a given material not only depends on the porosity of the material, but also on the temperature and relative humidity of the surrounding air. In damper environments, moisture levels in materials are higher than in drier environments. Obviously a material will absorb more moisture if it is exposed to rain.
The moisture content can also be greatly modified depending on what the material is in contact with. If a brick wall is constructed without a damp-proof course then moisture from the ground is absorbed into the brick through capillary action. Likewise, moisture can be absorbed by bricks if moist earth is in contact with the wall. Both these effects can also lead to rising damp. Note that as the moisture content of bricks increases, thermal resistance decreases and so thermal insulating properties are reduced.
Frost attack will be a potential problem where brick walls become saturated. Saturation can occur because of the failure of the design to protect brickwork or where an inappropriate brick type has been selected in an exposed position. It is also possible that individual bricks may be of poor quality, having been inadequately fired or because they contain impurities.
Boundary walls are particularly prone to frost attack. The sides and the top of wall are exposed to the elements and they are therefore easily saturated, particularly if the wall has inadequate copings. Exposure means that they are also subject to extremes of temperatures, including freezing conditions. The tops of walls often suffer the most because of radiative heat losses to the night air.
Roof overhangs and other key design details can offer protection from saturation. This protection will also guard against very low temperatures and in many cases the heat losses from inside the house will keep the temperature of the brickwork above freezing.
There have been cases where frost attack has occurred in older brick cavity walls following the insertion of thermal insulation in the wall cavity. Cavity insulation, by its very nature, lowers the temperature of the external leaf and restricts evaporation into the cavity.
Wall treatments such as the use of silicone (as a remedy for rain penetration) can also cause problems, because when applied inappropriately, they may inhibit the drying out of the brickwork and therefore increase the likelihood of frost attack.
[edit] Brick categories
For clay bricks there are three categories for frost resistance:
Bricks in the bottom category should not be used externally unless protected adequately from moisture, while Class F1 bricks should not be used in saturated conditions or where they are subject to repeated freezing and thawing. The water absorption of a brick is given as a percentage as follows:
[edit] Porosity
As the void content of a material increase so does its ability to absorb water by capillary action. The ability to absorb moisture depends on the size of voids and how accessible the voids are to the water at the surface. Timber, for example, has a large void content but surface water is absorbed quite slowly. In general, there is no direct correlation between porosity and other properties such as durability. Nevertheless, it is a useful quantity to calculate.
For a given material:
Alternatively, if the bulk density and solid density of the material is known:
Common bricks can absorb large amounts of water (up to around 20%) and many types are susceptible to frost damage. Engineering bricks absorb only about 6% and have excellent frost resistance. Although there are bricks which are frost resistant, even when they have high moisture levels, it is best to keep bricks as dry as possible before, during and after construction.
[edit] Efflorescence and staining
In some instances the appearance of bricks is affected by the development of efflorescence or stains. These may originate from materials in the brick or mortar, from adjacent materials or from outside sources such as cleaning agents. Each has a particular chemical composition and a unique means of removal.
Identification of the origin of the efflorescence, stain or foreign material is the first step in returning brickwork to its proper appearance. Stains are often misidentified or are mistaken for efflorescence. Since correctly identifying efflorescence or a stain can be difficult, it is recommended that experienced professionals verify the efflorescence or type of stain.
Mis-identification may result in application of an inappropriate correction method. When correctly identified, efflorescence and stains can generally be removed, whereas inappropriate correction methods may result in further staining or damage to the brickwork.
[edit] Efflorescence
Efflorescence is a common sight in new brickwork. It is caused by soluble salts in solution being brought to the surface as water in the wall dries out. It is usually a harmless, temporary problem, often occurring in spring following a wet winter. The main concern is the unsightly appearance caused by the white staining that it produces. Persistent efflorescence may indicate a design or construction fault that allows the brickwork to become, and to remain, saturated.
Efflorescence is caused by a number of soluble salts including the sulphate or carbonate compounds of calcium, sodium, potassium and magnesium. The salts may originate in the bricks or they may be introduced through the mixing water, cement or sand used for the mortar mix, or even from the ground on which the bricks were stacked and stored.
Additional sources may include sea air and unfortunate site practices such as the use of washing-up liquid as a mortar plasticiser, as washing-up liquid usually contains sodium chloride – common salt.
As the salts are water soluble they are often removed by rainfall, although they can usually be brushed off using a stiff brush if their appearance is causing concern.
Although it is usually a harmless problem there have been cases of damage caused by efflorescence. Crystallisation of salts just below the surface of the brick can cause spalling. This is known as cryptoflorescence. The problem is often associated with magnesium salts.
Cryptoflorescence is associated with a large build-up of salts and usually occurs where old, relatively weak, bricks are re-used inappropriately, particularly in areas of excessive dampness. It can also occur if the brickwork has been covered by a surface treatment because the salts may crystallise behind the treated surface and force it off. The effect on the bricks is similar to that caused by frost attack.
[edit] Lime run-off
Lime run-off is where excess water flows through cementatious material. Water can dissolve calcium hydroxide (free lime) which is then deposited on the brick face. The calcium hydroxide is a soluble form of lime which is created as Portland cement hydrates.
The source of the lime may be the cement from mortar joints or it may come from concrete or cast stone elements; for example, a coping above a brick wall or a floor slab built into the brickwork. Lime material washed from mortar joints can be due to lack of adequate protection against rainfall during construction.
The run-off is often seen ‘dribbling’ from weep holes or fine separation cracks between brick and mortar joints. The calcium hydroxide reacts with carbon dioxide in the air producing a hard crystalline formation of calcium carbonate. The initial staining can be removed with water and brushing before it carbonates but once reaction has taken placed an acid solution will be necessary.
For more information, see Lime run-off.
[edit] Other stains
[edit] Vanadium salts
These salts produce a yellow or green efflorescence in the heart of light-coloured brick on new brickwork. The salts occur naturally in certain clays (usually, but not exclusively those that are used to produce buff/lighter coloured bricks).
Vanadium stains occur in a similar manner to efflorescence, other than vanadium oxide and sulfates are dissolved and result in a solution that may be quite acidic. As water evaporates from this solution at the surface of the brickwork, vanadyl salts are deposited. The chloride salts of vanadium, such as vanadyl chloride, may form as a result of washing with unbuffered hydrochloric (muriatic) acid or excessive moisture exposure.
They are generally best left to the weather away naturally. However, preventing vanadium stains is important since they can be difficult to remove and improper cleaning efforts may result in a brown, insoluble deposit. To minimise the potential for vanadium stains, the following steps are recommended:
- Store bricks off the ground and under nonstaining protective covers.
- Never use or permit the use of highly-concentrated, unbuffered hydrochloric (muriatic) acid solutions to clean light-coloured brick.
- Seek and follow the cleaning recommendations of the brick manufacturer.
[edit] Iron staining
Iron staining usually appears as a stain to the mortar joint. The staining can come from metal imbedded in the structure or it can be derived from the brick or the mortar sand. Iron staining can be removed mechanically if the mortar is still relatively weak; otherwise chemicals may have to be employed. It if appears on the brick it should be allowed to weather away.
[edit] Manganese (brown) staining
Manganese staining manifests itself as a dark brown or black staining concentrated along mortar joints. It is caused by manganese dioxide (used as a colouring agent during manufacturing) that has dissolved in rainwater, construction water or muriatic acid.
During the brick firing process, the manganese colouring agents undergo several chemical changes, resulting in manganese compounds that are insoluble in water. They have varying degrees of solubility in weak acids. Once dissolved, these compounds may migrate in solution towards the surface of brickwork. As previously discussed, acid solutions can occur in brickwork under certain conditions. Brick may also absorb unbuffered hydrochloric (muriatic) acid during cleaning. It is also possible that some geographical areas may be subject to acid rain.
Manganese staining is closely related to efflorescence since it is the sulfate and chloride salts of manganese that travel to the surface of the brickwork. When the solution reaches the mortar joints, the salts are neutralised by the cement or lime in the mortar, producing insoluble manganese hydroxide. The manganese hydroxide precipitate is deposited on the mortar joint, and, when dry, converts to brown manganese tetroxide resulting in the stain.
Unbuffered hydrochloric (muriatic) acid should not be used to clean tan, brown, black or grey brick. Proprietary cleaning compounds are available for cleaning brick containing manganese. Test for effectiveness and follow the advice of the brick manufacturer.
[edit] White scum (silicate deposits)
Silicate deposits appear as uneven white or grey stain on the brick face or mortar joints. It often appears as vertical run marks, which do not disappear when wet. The cause is inadequate pre-wetting or rinsing when cleaning with muriatic acid or other acidic solutions. Mortar that is dissolved by the acid is absorbed by the dry wall surface to produce insoluble silicate salts commonly referred to as 'scumming'. White scum may also occur adjacent to trim elements, precast concrete and, occasionally, large expanses of glass. A specialist remover may be needed to clean the brickwork.
Silicate deposits on brick masonry should not be confused with scumming that sometimes occurs on bricks during the manufacturing process. This type of scumming will be evident on bricks before they are placed in the wall.
[edit] Acid burn
When cleaning brickwork with muriatic acid, the acid and impurities in the acid are rapidly absorbed by porous masonry and cannot be thoroughly water-rinsed. As the acid attacks the bricks and mortar, soluble and insoluble salts are mobilised to create unsightly and uneven yellow/gold staining on the brick face and in mortar joints. Stained areas may also exhibit serve etching or mortar discoloration. As with white scum staining, a specialist remover may be needed to clean the brickwork.
[edit] Stains from external sources
Other stains affecting brickwork are generally caused by external sources such as pollution, organic growth or runoff. Usually, the source and composition of these stains is obvious. Organic stains can include algae, mould or other organisms.
Certain materials above or adjacent to brickwork such as copper, bronze, aluminium, synthetic stucco or paint can stain brickwork surfaces. In addition, external stains can be cause by hard water from sprinkler systems. The colour and appearance should be considered during identification. Laboratory or field tests can determine the stain composition and assist in proper identification. Once the correct identification is made, the appropriate method of cleaning can be implemented.
Rust-coloured stains may actually be corrosion. Such stains can be the result of corrosion of wall ties or joint reinforcement in or adjacent to the brickwork. The use of improper mortar additives or ingredients, placement of wall ties or joint reinforcement with inadequate cover, welding splatter on the brick or the corrosion of a material placed on the brick cube or pile prior to being laid in the brickwork can all contribute to these stains.
[edit] Lime blow in bricks
This is caused when clay bricks contain small amounts of lime. When the bricks are fired, the lime is converted to calcium oxide (quicklime). When the bricks become wet the calcium oxide begins to slake. The process of slaking is vigorous and can cause an eruption on the face of the brick. Lime blow can also occur in plasters and renders.
[edit] Sulfate attack
Sulfate attack is a serious problem as it can cause crumbling of the mortar joint and expansion and instability in the wall. It is caused by a reaction between sulfates in solution and a constituent of Ordinary Portland Cement known as tricalcium aluminate.
Sulfate attack relies upon a number of conditions occurring simultaneously; it requires water saturation over a relatively long period, a source of sulfates and reasonable amount of tricalcium aluminate. Even where these factors exist simultaneously the attack will take a relatively long time to develop. The rate of deterioration is affected by the quantity and the type of sulfate – the sulfates of magnesium and potassium are the most aggressive.
The reaction between the sulfates and the tricalcium aluminate forms a compound known as calcium sulfo-aluminate. This compound expands as it forms, leading to cracking in the mortar joints, followed by general deterioration and the loss of the mortar’s ‘bonding’ function, as the face of the joint spalls and the mortar cracks and crumbles. The cracks can be at the edge of the mortar joint or through the middle. The expansion, which leads to leaning and bulging, exacerbates the instability in the brickwork caused by the deterioration of the mortar. Sometimes the faces of the bricks spall, most commonly around their edges.
As they are being exposed on both sides, parapet walls are at high risk from sulfate attack. They can be recognised by pronounced cracks in the bed joints. This should not be confused with wall tie failure.
The source of sulfate can be the bricks themselves, but they can also be introduced from the ground or from air pollution. An additional source is the exhaust gases from slow-burning fuel appliances. Sulfate attack usually occurs in situations which are particularly exposed to relatively large amounts of water.
A common consequence of sulfate attack in chimneys is a pronounced lean caused by the different wetting and drying cycles between different elevations. In chimneys additional sulfate may be deposited by the combustion process and additional water may be introduced from condensation within the chimney itself.
The situation is often made worst by applying render to ‘at risk’ elements. Render which is too strong (i.e. has too much cement in it) may shrink and allow rainwater into the wall. Because of its density, a strong render restricts the rate of evaporation. As sulfate attack will also cause cracking in the render, more water can get in and this further exacerbates the situation.
The horizontal cracking caused by sulfate attack can be distinguished from that caused by wall tie failure (discussed below) because it may occur in every joint. Also there is often a characteristic white colouring to the mortar as it deteriorates. As the sulfate attack involves water saturation, it is often accompanied by frost attack.
[edit] Wall tie failure
Main article: Wall Tie Failure
Failure of wall ties has become a significant problem in recent years. The main cause of failures is rusting of metal ties, although there can be other causes, such as failure to properly bed the tie in the mortar joint, poor quality mortar reducing the bond between tie and mortar, or not installing the requisite number of ties.
The obvious danger with rusting wall ties is the possible collapse of the outer leaf of the cavity wall. Other consequences of rusting wall ties are:
- The rust will have a significantly greater volume than the original metal. This expansion of the tie may cause cracking and distortion of the structure, particularly where strip ties have been used. The rust-induced expansion in strip ties can lead to secondary damage, such as a redistribution of loads, buckling and bulging of wall, and damage to the roof as the external leaf increases in height.
- The less bulky wall ties will not generally produce enough expansion to induce cracking unless the joint is abnormally thin or the mortar is very dense. Unfortunately, wire ties produced in the UK before 1981 had less rust protection than strip ties and therefore are likely to have a shorter life expectancy; a particular problem because failure can occur without the outwardly visible warning signs produced by cracking.
- Cracking will also reduce the weather resistance of the wall, which in turn accelerates the rusting process.
[edit] Weathering and disintegration of joints
Mortar can be finished with one of a number of different profiles. The profile of a joint has an aesthetic effect – because of the way it casts shadows and reflects light – and it affects the durability of a wall. Some profiles are more effective than others in preventing water ingress which can lead to frost damage. The nature of a joint can be more significant than the porosity of the brick in terms of weather protection.
- A tooled joint using a bucket handle is effective. The tooling compresses the mortar slightly, thus reducing its permeability. At the same time tooling ensures a good seal between mortar and brick.
- Struck joints are equally effective although they require slightly more skill.
- Recessed joints are problematical because they do not easily shed rainwater but allow it to sit on the edge of the brick and, although tooling may compress and seal the joint, the brick is at high risk. Recessed joints should only be used on brickwork that is not exposed to rain and with bricks that are excellent at resisting frost damage, e.g. engineering bricks.
- Flush joints often leave small cracks between the brick and the mortar. Also, the mortar is often ‘buttered over’ (mortar on the face of the bricks) the joint, increasing the likelihood of water penetration.
[edit] Related articles on Designing Buildings
- Basic brickwork replacement.
- Blockwork.
- Brick.
- Condensation.
- Cracking and building movement.
- Cracking in buildings.
- Dabs.
- Damp proof course.
- Defects in dot and dab.
- Defects in stonework.
- Does damp proofing work?
- Efflorescence.
- How to lay bricks.
- Lime run-off.
- Mortar.
- Mould growth.
- Parapet.
- Pattern staining and soiling in stonework.
- Penetrating damp.
- Practical Building Conservation: Earth, Brick and Terracotta.
- Preventing wall collapse.
- Repointing.
- Reversible and irreversible expansion.
- Rising damp.
- Rising damp in walls - diagnosis and treatment (DG 245).
- Settlement.
- Slaking.
- Spalling.
- Specifying brick.
- Stain.
- Thermal expansion.
- Treating brickwork with sealant or water repellent.
- Types of brick bonding.
- Understanding dampness.
- Wall tie failure.
- Which way up should you lay a brick?
- Why do buildings crack? (DG 361).
IHBC NewsBlog
Latest IHBC Issue of Context features Roofing
Articles range from slate to pitched roofs, and carbon impact to solar generation to roofscapes.
Three reasons not to demolish Edinburgh’s Argyle House
Should 'Edinburgh's ugliest building' be saved?
IHBC’s 2025 Parliamentary Briefing...from Crafts in Crisis to Rubbish Retrofit
IHBC launches research-led ‘5 Commitments to Help Heritage Skills in Conservation’
How RDSAP 10.2 impacts EPC assessments in traditional buildings
Energy performance certificates (EPCs) tell us how energy efficient our buildings are, but the way these certificates are generated has changed.
700-year-old church tower suspended 45ft
The London church is part of a 'never seen before feat of engineering'.
The historic Old War Office (OWO) has undergone a remarkable transformation
The Grade II* listed neo-Baroque landmark in central London is an example of adaptive reuse in architecture, where heritage meets modern sophistication.
West Midlands Heritage Careers Fair 2025
Join the West Midlands Historic Buildings Trust on 13 October 2025, from 10.00am.
Former carpark and shopping centre to be transformed into new homes
Transformation to be a UK first.
Canada is losing its churches…
Can communities afford to let that happen?
131 derelict buildings recorded in Dublin city
It has increased 80% in the past four years.








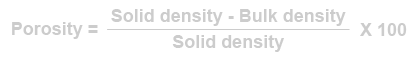














Comments
To start a discussion about this article, click 'Add a comment' above and add your thoughts to this discussion page.