Silicon
Contents |
[edit] Introduction
Silicon (chemical symbol Si) is a naturally occurring, non-metallic element and, after oxygen, is the second most abundant element in the earth’s crust, found mainly as silica in sand. Silicates are the chief constituents of many rocks, clays and soils and make up more than 90% of the Earth’s crust.
Hard and brittle, silicon has a crystalline structure and a metallic blue-grey lustre. Because it is classed as ‘metalloid’, it displays properties of both metals and non-metals.
Silicon has an affinity for oxygen and so is rarely found in its pure elemental form but as silicon dioxide (SiO2 or silica). Silica occurs in 12 different crystal modifications which include alpha quartz, a main constituent of granite and sandstone, rock crystal, rose quartz, smoky quartz, morion, amethyst and citrine. Also, as agate, onyx, jasper and flint.
Commercially, silicon is used mostly without processing in semiconductors, and is essential to integrated circuits. Its high conductivity also sees its use in solar power cells. But its main use is in steel refining, aluminium casting and chemicals. Silicon of up to 99% purity can be made using an electric arc furnace by reducing quartzite or sand with high-purity coke
[edit] Uses in construction
Many construction materials contain silica e.g asphalt, brick, cement, concrete, plasterboard, grout, mortar, tile and stone. It is also used as a filler in some plastics and is added to white ceramic ware such as porcelain and to some types of glass.
Silicates are used in the production of Portland cement for concrete, mortar and stucco, and also form the basis for the widely-used synthetic polymers called silicones. Silicates are also constituents of optical fibres, fibreglass and glass wool for thermal insulation.
Silicon resins are used in the construction industry as additions to coatings to which they impart resistance to heat, oils, salts, acids, and alkalis. Because they also provide water repellence, they are used in water repellent treatments for brickwork and masonry.
Silicon is also added to polishes, mechanical seals, high temperature greases and waxes, caulking compounds, breast implants, contact lenses, explosives and fireworks. Also, to hi-tech abrasives, hi-strength ceramics and in super alloys.
[edit] Silicone rubber
Silicone rubber is a synthetic rubber. Compared to natural rubber, it has better resistance to high and low temperatures.
[edit] Silicon carbide
This is used as an abrasive to smooth a variety of materials. It is formed when carbon and sand (or silica) are heated together in a furnace. It may be marketed under the trade name of ‘carborundum’.
[edit] Silicosis
Exposure to silica dust can pose a major health hazard and lead to silicosis of the lung or ‘pneumonoultramicroscopicsilicovolcanoconiosis’. It is a long-term disease usually caused by inhaling large amounts of crystalline silica dust, typically over many years.
The Health & Safety Executive’s ‘Control of Exposure to Silica Dust’ advises that when cutting, sanding or carving materials containing silica, a fine dust (Respirable Crystalline Silica (RCS)) is created that may get into the lungs. RCS is too fine to see under normal lighting conditions.
Typical activities that may generate RCS include:
- Construction and demolition processes – concrete, stone, brick, mortar;
- Quarrying;
- Slate mining and slate processing;
- Potteries, ceramics, ceramic glaze manufacture, brick and tile manufacture;
- Foundries;
- Refractory production and cutting;
- Concrete product manufacture;
- Monumental and architectural masonry manufacture, stone fireplace and
- Kitchen worktop manufacture, and
- Grit and abrasive blasting, particularly on sandstone.
For more information see: Silica.
[edit] Silicon, silicone and silica
Differentiating between silicon, silicone and silica
- Silicon is a naturally occurring element
- Silicone is a man-made polymer that is derived from silicon, a class of silicon-based chemical compounds used in paints, adhesives, lubricants and breast implants, among other applications
- Silica is another name for silicon dioxide (SiO2).
[edit] Related articles on Designing Buildings
- Caulk v silicone sealant.
- Concrete superplasticizer.
- Fabric structures.
- Glass.
- Laitance.
- Plastic cladding.
- Plastic.
- Polycarbonate plastic.
- Polymers.
- Putty.
- Rubber.
- Silica.
- Spalling.
- The development of structural membranes.
[edit] External links
Featured articles and news
Building Safety recap January, 2026
What we missed at the end of last year, and at the start of this...
National Apprenticeship Week 2026, 9-15 Feb
Shining a light on the positive impacts for businesses, their apprentices and the wider economy alike.
Applications and benefits of acoustic flooring
From commercial to retail.
From solid to sprung and ribbed to raised.
Strengthening industry collaboration in Hong Kong
Hong Kong Institute of Construction and The Chartered Institute of Building sign Memorandum of Understanding.
A detailed description fron the experts at Cornish Lime.
IHBC planning for growth with corporate plan development
Grow with the Institute by volunteering and CP25 consultation.
Connecting ambition and action for designers and specifiers.
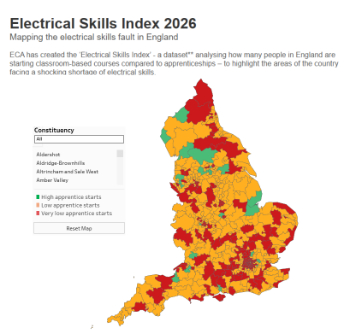
Electrical skills gap deepens as apprenticeship starts fall despite surging demand says ECA.
Built environment bodies deepen joint action on EDI
B.E.Inclusive initiative agree next phase of joint equity, diversity and inclusion (EDI) action plan.
Recognising culture as key to sustainable economic growth
Creative UK Provocation paper: Culture as Growth Infrastructure.
Futurebuild and UK Construction Week London Unite
Creating the UK’s Built Environment Super Event and over 25 other key partnerships.
Welsh and Scottish 2026 elections
Manifestos for the built environment for upcoming same May day elections.
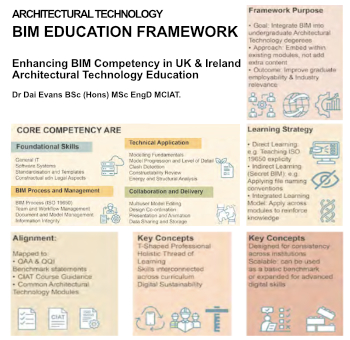
Advancing BIM education with a competency framework
“We don’t need people who can just draw in 3D. We need people who can think in data.”
Guidance notes to prepare for April ERA changes
From the Electrical Contractors' Association Employee Relations team.
Significant changes to be seen from the new ERA in 2026 and 2027, starting on 6 April 2026.
First aid in the modern workplace with St John Ambulance.
Solar panels, pitched roofs and risk of fire spread
60% increase in solar panel fires prompts tests and installation warnings.
Modernising heat networks with Heat interface unit
Why HIUs hold the key to efficiency upgrades.























Comments
[edit] To make a comment about this article, click 'Add a comment' above. Separate your comments from any existing comments by inserting a horizontal line.