Marine corrosion
Contents |
[edit] Introduction
A passenger ship that can accommodate up to 500 passengers can cost an average of $260 million. This staggering amount places importance on the timely and adequate maintenance of such vessels. That’s why billions are spent every year to keep corrosion under control.
Ships and other marine vessels are in constant contact with water and air that are concentrated with salts. Dealing with corrosion is one of the major challenges faced by the marine industry. Inadequate preventive measures can lead to total asset loss, significant maintenance costs, premature failures, and reduced service life.
Depending on construction metals, vessels are susceptible to different degrees of corrosion. For instance, galvanized steel is much less resistant to corrosion than some grades of stainless steel. Corrosion also depends on exposure to different marine environments. The worse the environmental conditions, the worse will be the impact of corrosion.
[edit] Types of corrosion
Some commonly found forms of corrosion include:
- Uniform corrosion. One of the most common forms of corrosion, uniform corrosion evenly corrodes the entire surface area. Without any preventive measure, it will continue to thin out the surface until eventual failure.
- Crevice corrosion. Crevice corrosion occurs where two or more metal or non-metallic materials are joined. It usually takes place in confined spaces where elements like oxygen are limited.
- Pitting corrosion. Pitting corrosion is localized corrosion often caused by exposure to aggressive chemicals such as chloride. It is considered far more dangerous than uniform corrosion as its detection can be difficult.
- Flow-accelerated corrosion. A ship constantly moves through water that has an adverse effect on its structure. This is a form of flow-accelerated corrosion. The friction eventually degrades the outer protective layer to expose the metal underneath, causing further corrosion.
- Microbiologically-induced corrosion. Microbiologically-induced corrosion is caused by microorganisms such as chemoautotrophs. This localised corrosion can lead to leaks and pinholes.
- Stress corrosion cracking. When a crack is exposed to tensile stress, it expands and can lead to unexpected failure of otherwise ductile alloy metals.
- Intergranular cracking. A crystallised surface is more susceptible to corrosion than its insides. This results in intergranular fractures along the boundaries. Tests can be performed to discover a material’s vulnerability to intergranular cracking.
- Galvanic corrosion. Galvanic corrosion occurs when two or more metals that are in contact with each other and submerged in concentrated salt water.
There are several ways to manage and prevent corrosion and prolong overall service life.
[edit] Coating systems
Putting a durable coating system in place will act as a barrier between the ship and corrosive elements i.e, moisture and oxygen. Applying thick coats of industrial-grade paint is one of the most common ways to keep corrosion at bay. It is especially necessary for the part of the ship that remains below the sea level. Usually, a combination of coal-tar epoxy and vinyl tar is used to minimise the exposure to salts and minerals found in environmental elements.
From the hull of the ship to the deck, no area is safe from water, especially during rough seas. Typically, chlorinated rubber coatings and an alkyd are used for this purpose.
In addition to corrosion prevention, coating systems also assist vessels by reducing friction. Coating systems are imperative to extend the life of a ship and save on maintenance costs. Keeping up paintwork requires effort, but as long as it is in good condition without any cracks, it is an excellent way to avert corrosion.
So how does the marine industry ensure the coating systems are doing their job? They conduct Coating Inspection and Surveying to make sure that the highest levels of coating application standards and specifications are met.
[edit] Cathodic inhibitors
This is another popular technique used to protect metals from corrosion. It involves making the metal surface the cathode of an electrochemical cell. It is used for pipelines, ship hulls, storage tanks, water heaters and so on.
[edit] Galvanized anodes
These are often used inside the ship and sometimes on parts of the hull. Essentially, this entails attaching an anode to a vulnerable surface that’s exposed to an electrolyte. Such an anode is allowed to corrode freely, while the structure remains protected.
Sacrificial anodes are commonly made from magnesium, aluminium and alloys of zinc. They can be constructed in all shapes and sizes to fit a structure’s dimensions. Over time, the anode material completely corrodes and has to be replaced.
[edit] Impressed current
Impressed current is quite similar to sacrificial anodes. The only difference being, instead of a metal, DC power supply does the job of an anode. The power supply is used to curb the chemical reaction between metals and corrosive elements. This is prefered over sacrificial anodes as it requires low maintenance. Corrosion will remain in check as long as there is sufficient power supply
[edit] Hybrid systems
Hybrid systems are a combination of the above two. They are made of galvanised anodes powered by high restorative current flow.
[edit] Strict maintenance procedure
Regular and timely maintenance is important to keep corrosion under control. This includes careful inspection for any damage and prompt rectification to ensure it doesn’t become worse. Rather than assigning maintenance in-house, many prefer to outsource it for efficiency and cost-effectiveness.
[edit] Related articles on Designing Buildings Wiki
Featured articles and news
What they are, how they work and why they are popular in many countries.
Plastic, recycling and its symbol
Student competition winning, M.C.Esher inspired Möbius strip design symbolising continuity within a finite entity.
Do you take the lead in a circular construction economy?
Help us develop and expand this wiki as a resource for academia and industry alike.
Warm Homes Plan Workforce Taskforce
Risks of undermining UK’s energy transition due to lack of electrotechnical industry representation, says ECA.
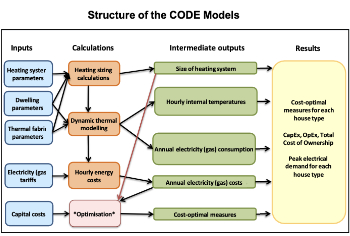
Cost Optimal Domestic Electrification CODE
Modelling retrofits only on costs that directly impact the consumer: upfront cost of equipment, energy costs and maintenance costs.
The Warm Homes Plan details released
What's new and what is not, with industry reactions.
Could AI and VR cause an increase the value of heritage?
The Orange book: 2026 Amendment 4 to BS 7671:2018
ECA welcomes IET and BSI content sign off.
How neural technologies could transform the design future
Enhancing legacy parametric engines, offering novel ways to explore solutions and generate geometry.
Key AI related terms to be aware of
With explanations from the UK government and other bodies.
From QS to further education teacher
Applying real world skills with the next generation.
A guide on how children can use LEGO to mirror real engineering processes.
Data infrastructure for next-generation materials science
Research Data Express to automate data processing and create AI-ready datasets for materials research.
Wired for the Future with ECA; powering skills and progress
ECA South Wales Business Day 2025, a day to remember.
AI for the conservation professional
A level of sophistication previously reserved for science fiction.
Biomass harvested in cycles of less than ten years.
An interview with the new CIAT President
Usman Yaqub BSc (Hons) PCIAT MFPWS.
Cost benefit model report of building safety regime in Wales
Proposed policy option costs for design and construction stage of the new building safety regime in Wales.
Do you receive our free biweekly newsletter?
If not you can sign up to receive it in your mailbox here.