Electrochemical cell
There are generally two main types of electrochemical cells: primary (non-rechargeable) and secondary (rechargeable) batteries.
A galvanic cell also called a voltaic cell, is an electrochemical cell in which an electric current is generated from an oxidation-reduction reaction. The cell normally consists of two different metals (electrodes), immersed in separate containers that hold a positively or negatively charged solution. A salt bridge or porous membrane between the containers transfers charge. Energy is derived from a high-cohesive-energy metal dissolving while to a lower-energy metal is deposited, and/or from high-energy metal ions plating out while lower-energy ions go into solution. A primary or non-rechargeable battery is a galvanic cell, a secondary or rechargeable battery acts as a galvanic cell when it is discharging, as it is converting chemical energy to electrical energy. A secondary or rechargeable battery acts as an electrolytic cell when it is being charged as in this case it is converting electrical energy to chemical energy.
An electrolytic cell, is one in which a current is passed through by an external voltage, causing a chemical reaction. An electrolytic cell has three parts: a cathode, an anode (electrodes) and an electrolyte. As above electrolyte is usually a solution of water or another solvent, such as sodium chloride with dissolved ions. When an external voltage is applied to the electrodes, the ions in the electrolyte are attracted to an electrode with the opposite charge, the charge-transferring causes the chemical reaction, faradaic or redox. In the galvanic cell the chemical reaction causes electric current to flow.
An equilibrium electrochemical cell is a cell that sits in a state that is directly between the two above cell states, so nether storing chemical energy nor releasing electrical energy.
A fuel cell is considered to be a galvanic cell but because the products of the reaction are continuously removed it is one that requires a supply of reactants. It does not therefore store chemical or electrical energy but extracts electrical energy directly from a chemical reaction involving oxygen, hydrogen, electricity, heat and water.
[edit] Related articles on Designing Buildings
Featured articles and news
A case study and a warning to would-be developers
Creating four dwellings... after half a century of doing this job, why, oh why, is it so difficult?
Reform of the fire engineering profession
Fire Engineers Advisory Panel: Authoritative Statement, reactions and next steps.
Restoration and renewal of the Palace of Westminster
A complex project of cultural significance from full decant to EMI, opportunities and a potential a way forward.
Apprenticeships and the responsibility we share
Perspectives from the CIOB President as National Apprentice Week comes to a close.
The first line of defence against rain, wind and snow.
Building Safety recap January, 2026
What we missed at the end of last year, and at the start of this...
National Apprenticeship Week 2026, 9-15 Feb
Shining a light on the positive impacts for businesses, their apprentices and the wider economy alike.
Applications and benefits of acoustic flooring
From commercial to retail.
From solid to sprung and ribbed to raised.
Strengthening industry collaboration in Hong Kong
Hong Kong Institute of Construction and The Chartered Institute of Building sign Memorandum of Understanding.
A detailed description from the experts at Cornish Lime.
IHBC planning for growth with corporate plan development
Grow with the Institute by volunteering and CP25 consultation.
Connecting ambition and action for designers and specifiers.
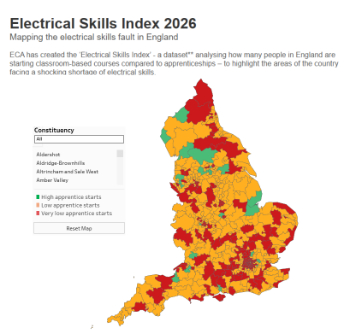
Electrical skills gap deepens as apprenticeship starts fall despite surging demand says ECA.
Built environment bodies deepen joint action on EDI
B.E.Inclusive initiative agree next phase of joint equity, diversity and inclusion (EDI) action plan.
Recognising culture as key to sustainable economic growth
Creative UK Provocation paper: Culture as Growth Infrastructure.