Types and colours of hydrogen
Contents |
[edit] What is hydrogen?
Hydrogen (H) is a chemical element with atomic number 1. It is the most abundant chemical substance in the universe. At standard temperature and pressure it occurs as a colourless, odourless, tasteless gas H2 which is highly combustible. It is suitable as a fuel, for example to supply heat and power to buildings. Its production processes and associated emissions vary dramatically, and so the different methods are are colour coded to describe their footprint.
Net Zero by 2050, A Roadmap for the Global Energy Sector, published by the International Energy Agency in May 2021, states: ‘Hydrogen is used in the energy system to refine hydrocarbon fuels and as an energy carrier in its own right. It is also produced from other energy products for use in chemicals production. As an energy carrier it can be produced from hydrocarbon fuels or from the electrolysis of water with electricity, and can be burned or used in fuel cells for electricity and heat in a wide variety of applications. To be low-carbon hydrogen, either the emissions associated with fossil-based hydrogen production must be prevented (for example by carbon capture, utilisation and storage) or the electricity input to hydrogen produced from water must be low-carbon electricity. In this report, final consumption of hydrogen includes demand for pure hydrogen and excludes hydrogen produced and consumed onsite by the same entity. Demand for hydrogen-based fuels such as ammonia or synthetic hydrocarbons are considered separately.’
Hydrogen-based fuels include ammonia and synthetic hydrocarbons.
A number of hydrogen production techniques, and resulting hydrogen types are described below.
[edit] Hydrogen production through electrolysis
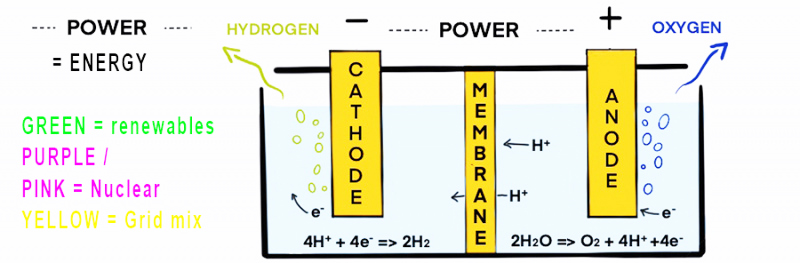
Most hydrogen is produced using electricity is via electrolysis. Electrolysis uses two electrodes which are immersed in water containing salts and minerals, which help electrical conduction. The electrodes attract ions with an opposite charge, separating the hydrogen and oxygen, an oxidation-reduction reaction occurs as a result of the electricity.
The key variation in this type of hydrogen production is the source of the electricity. The hydrogen coded by colour to indicate the source of the energy.
[edit] Green hydrogen
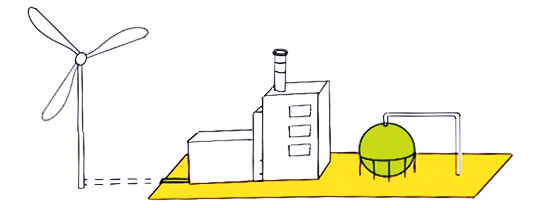
Green hydrogen is produced through electrolysis using renewable energy sources such as wind, solar, hydroelectric, geothermal or tidal energy. In general, because of the zero emissions of the energy sources this type of hydrogen production has a low carbon footprint.
[edit] Purple / pink hydrogen
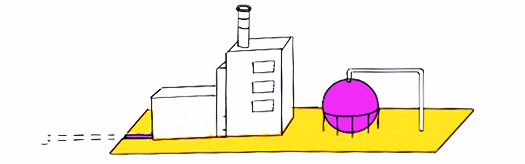
Purple / pink hydrogen is produced through electrolysis using nuclear energy sources. In general because of the zero emissions of the energy sources, this type of hydrogen production is considered to have a low carbon footprint (consider though nuclear waste issues etc).
[edit] Yellow hydrogen
Yellow hydrogen is produced through electrolysis using grid energy which has a varied mix of origins. In general because the energy source is from a standard grid mix, its impact will depend somewhat on the mix of the specific grid being used to provide the electricity, but this type of hydrogen production is likely to have a medium to high carbon footprint reducing as the grid energy supply moves to renewables.
[edit] Hydrogen production with fossil fuels
[edit] Blue hydrogen
Blue hydrogen production uses natural gas reforming. Methane reacts with steam under temperature and pressure in the presence of a catalyst to produce hydrogen, carbon monoxide, and a small amount of carbon dioxide. The hydrogen is used as a fuel and the carbon dioxide can be captured, utilised and stored (CCUS). The by-product of the process can be solid carbon. The fuels used to carry out the process are usually natural gas or coal (which would push it towards being more black or at least dark blue hydrogen).
[edit] Turquoise hydrogen
Turquoise hydrogen production uses pyrolysis and natural gas as a fuel source. Another option for hydrogen production with potentially low greenhouse gas emissions is the decomposition of methane from natural gas sometimes referred to as pyrolysis. This process decomposes methane into its elements; hydrogen and solid carbon (CH4 → C + 2 H2).
[edit] Grey hydrogen
Grey hydrogen production uses natural gas reforming. Over half the worlds hydrogen production and over 90% of US hydrogen is made by natural gas reforming, the primary form of which is steam-methane reforming, which can also use other fuels such as ethanol, propane, and gasoline. A second process, called partial oxidation, can also be used though this is not as common.
Steam-methane reforming, is where methane reacts with steam under pressure in the presence of a catalyst to produce hydrogen, carbon monoxide, and a small amount of carbon dioxide. It is an endothermic reaction so heat must be provided for reaction to occur, unlike partial oxidation which is an exothermic reaction. Carbon monoxide and steam react using a catalyst to produce carbon dioxide and more hydrogen (called the water-gas shift reaction), then carbon dioxide and other impurities are removed leaving pure hydrogen (pressure-swing adsorption). There are medium level emissions associated with this procedure due to the energy used to provide the heat required.
[edit] Brown hydrogen
Brown hydrogen production uses gasification where the fuel source used is brown coal (lignite). Gasification is a process that converts organic or fossil-based carbonaceous materials into carbon monoxide, hydrogen, and carbon dioxide. It does this by heating the material to very high temperatures in controlled amounts of oxygen and steam without combustion occurring. Carbon monoxide reacts with water to form carbon dioxide and hydrogen (water-gas shift reaction) which is separated by absorbers or membranes.
[edit] Black hydrogen
Black hydrogen production uses gasification where the fuel source used is black coal. Gasification is a process that converts organic or fossil-based carbonaceous materials into carbon monoxide, hydrogen, and carbon dioxide, it does so by heating the material to very high temperatures in controlled amounts of oxygen and steam without combustion occurring. Carbon monoxide reacts with water to form carbon dioxide and hydrogen (water-gas shift reaction) which is separated by absorbers or membranes.
[edit] Cost comparisons
Although it is difficult to allocate direct costs to each of the different process, because technologies are developing very rapidly, the European Commission's hydrogen strategy of July 2020 made the following comparisons.
Green hydrogen produced with renewable resources costed between about $3/kg and $6.55/kg, whilst blue hydrogen, paired with carbon capture and steam methane reformation of natural gas, was about $2.40/kg, which compares to $1.80/k for fossil-based hydrogen.
However in 2021 due to reductions in the costs of renewable energy supplies these prices are likely to fall, for example a Norwegian electrolyzer-maker announced the goal of producing green hydrogen at $1.50 per kilogram by 2025, whilst in Malaysia by targeting hydrogen production costs from the nation's hydropower and solar resources a range of $1/kg to $2/kg is being targetted.
[edit] Related articles on Designing Buildings
- Blue hydrogen
- Carbon capture and storage.
- Clean hydrogen
- ECA responds to the UK hydrogen strategy.
- Hydrogen embrittlement
- Hydrogen for heat
- Is hydrogen for heating the fuel of the future?
- Low carbon hydrogen
- Offshore generation of hydrogen at far-from-shore wind farms
- Planning now for hydrogen
- Types of fuel.
[edit] External references
- HM Government, UK Hydrogen Strategy.
- https://www.researchgate.net/figure/The-hydrogen-color-spectrum-and-indications-for-carbon-emissions-11_fig1_358515834
- https://www.energy.gov/eere/fuelcells/hydrogen-and-fuel-cell-technologies-office
- Robert W Howarth of Cornell University and Mark Z Jacobson, Energy Science & Engineering, ‘How green is blue hydrogen?’
Featured articles and news
A case study and a warning to would-be developers
Creating four dwellings... after half a century of doing this job, why, oh why, is it so difficult?
Reform of the fire engineering profession
Fire Engineers Advisory Panel: Authoritative Statement, reactions and next steps.
Restoration and renewal of the Palace of Westminster
A complex project of cultural significance from full decant to EMI, opportunities and a potential a way forward.
Apprenticeships and the responsibility we share
Perspectives from the CIOB President as National Apprentice Week comes to a close.
The first line of defence against rain, wind and snow.
Building Safety recap January, 2026
What we missed at the end of last year, and at the start of this...
National Apprenticeship Week 2026, 9-15 Feb
Shining a light on the positive impacts for businesses, their apprentices and the wider economy alike.
Applications and benefits of acoustic flooring
From commercial to retail.
From solid to sprung and ribbed to raised.
Strengthening industry collaboration in Hong Kong
Hong Kong Institute of Construction and The Chartered Institute of Building sign Memorandum of Understanding.
A detailed description from the experts at Cornish Lime.
IHBC planning for growth with corporate plan development
Grow with the Institute by volunteering and CP25 consultation.
Connecting ambition and action for designers and specifiers.
Electrical skills gap deepens as apprenticeship starts fall despite surging demand says ECA.
Built environment bodies deepen joint action on EDI
B.E.Inclusive initiative agree next phase of joint equity, diversity and inclusion (EDI) action plan.
Recognising culture as key to sustainable economic growth
Creative UK Provocation paper: Culture as Growth Infrastructure.



















Comments
[edit] To make a comment about this article, click 'Add a comment' above. Separate your comments from any existing comments by inserting a horizontal line.
So hydrogen isnt just hydrogen then - who knew. Really interesting read.