Calorific value
The term ‘calorific value’ (CV) is a measure of heating power, and refers to the amount of energy released when a fuel is completely combusted under specific conditions. For solid and liquid fuels this is measured at constant volume and for gaseous fuels it is measured at constant pressure. See also Gross Heat of Combustion.
Calorific vales for a range of fuels are published by the UK government.
Typically, calorific vales are expressed in Megajoules per cubic metre (MJ/m3) for solid and liquid fuels or Megajoules per kilogram (MJ/kg) for gaseous fuels.
Calorific value can be expressed as a Net Calorific Value (NCV, or Lower Heating Value), or a Gross Calorific Value (GCV, a Higher Heating Value):
- The Net Calorific Value considers that the combustion products contain water of combustion to the vapour state, and so the heat energy in the water is not recovered.
- The Gross Calorific Value considers that the water of combustion is condensed, and so the heat energy in the water is recovered.
NB Embodied Carbon, The Inventory of Carbon and Energy (ICE), By Prof. Geoffrey Hammond and Craig Jones, Ed. Fiona Lowrie and Peter Tse, published by BSRIA in 2011, defines the Calorific Value (CV) of energy as: ‘The energy content of a fuel (as may be released through combustion). It may be expressed as a gross calorific value (GCV) or net calorific value (NCV). The former is always larger than (or equal to) the latter. The difference is due to latent heat (energy) remaining in condensation (water vapour) after combustion. The difference is typically 5-10 per cent (e.g. 10 per cent for natural gas, 5 per cent for coal).’
Combined heat and power quality assurance (CHPQA) guidance notes, published by the Department for Business, Energy & Industrial Strategy in 2014, suggests that the: ‘Gross Calorific Value (GCV) of a fuel is the total energy available from that fuel (solid, liquid or gas) when it is completely burnt. It is expressed as heat per unit weight or volume of fuel. ‘Gross’ signifies that the water formed or liberated during combustion is condensed to the liquid phase. The GCV of a solid or liquid fuel is determined at constant volume and the GCV of a gaseous fuel is determined at constant pressure.’
[edit] Related articles on Designing Buildings
Featured articles and news
Apprenticeships and the responsibility we share
Perspectives from the CIOB President as National Apprentice Week comes to a close.
The first line of defence against rain, wind and snow.
Building Safety recap January, 2026
What we missed at the end of last year, and at the start of this...
National Apprenticeship Week 2026, 9-15 Feb
Shining a light on the positive impacts for businesses, their apprentices and the wider economy alike.
Applications and benefits of acoustic flooring
From commercial to retail.
From solid to sprung and ribbed to raised.
Strengthening industry collaboration in Hong Kong
Hong Kong Institute of Construction and The Chartered Institute of Building sign Memorandum of Understanding.
A detailed description from the experts at Cornish Lime.
IHBC planning for growth with corporate plan development
Grow with the Institute by volunteering and CP25 consultation.
Connecting ambition and action for designers and specifiers.
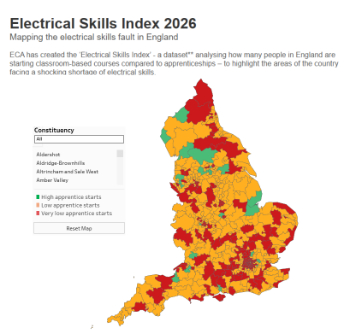
Electrical skills gap deepens as apprenticeship starts fall despite surging demand says ECA.
Built environment bodies deepen joint action on EDI
B.E.Inclusive initiative agree next phase of joint equity, diversity and inclusion (EDI) action plan.
Recognising culture as key to sustainable economic growth
Creative UK Provocation paper: Culture as Growth Infrastructure.
Futurebuild and UK Construction Week London Unite
Creating the UK’s Built Environment Super Event and over 25 other key partnerships.
Welsh and Scottish 2026 elections
Manifestos for the built environment for upcoming same May day elections.
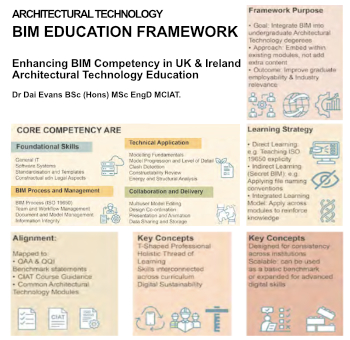
Advancing BIM education with a competency framework
“We don’t need people who can just draw in 3D. We need people who can think in data.”




















Comments
[edit] To make a comment about this article, or to suggest changes, click 'Add a comment' above. Separate your comments from any existing comments by inserting a horizontal line.