Water vapour in the built environment
Water vapour is the phase of water (H2O) that takes the form of a gas. It is a component of the Earth’s hydrologic cycle and is abundant in the atmosphere.
Boiling liquid water can produce water vapour by a process of evaporation, while ice can produce water vapour via sublimation. The use of water vapour in the form of steam has been a fundamental part of energy production since the Industrial Revolution.
Air will generally include moisture in the form of water vapour. Absolute humidity is the mass of water vapour in a volume of air divided by the mass of dry air. When air cools, it is less able to 'hold' moisture, that is, the saturation water vapour density falls, and so the relative humidity rises. When the relative humidity reaches 100%, the air will be saturated. This is described as the dew point. If the air continues to cool, moisture will begin to condense.
For more information see: Humidity.
The presence of water vapour in a building can lead to problems with condensation and moisture which can result in mould growth, damp, staining, and so on.
Moisture in buildings can be reduced by removing or reducing the source of water (such as washing processes, leaking pipes, rising damp and so on) or by drainage, ventilation, increasing temperatures or dehumidification.
[edit] Related articles on Designing Buildings
Featured articles and news
A case study and a warning to would-be developers
Creating four dwellings... after half a century of doing this job, why, oh why, is it so difficult?
Reform of the fire engineering profession
Fire Engineers Advisory Panel: Authoritative Statement, reactions and next steps.
Restoration and renewal of the Palace of Westminster
A complex project of cultural significance from full decant to EMI, opportunities and a potential a way forward.
Apprenticeships and the responsibility we share
Perspectives from the CIOB President as National Apprentice Week comes to a close.
The first line of defence against rain, wind and snow.
Building Safety recap January, 2026
What we missed at the end of last year, and at the start of this...
National Apprenticeship Week 2026, 9-15 Feb
Shining a light on the positive impacts for businesses, their apprentices and the wider economy alike.
Applications and benefits of acoustic flooring
From commercial to retail.
From solid to sprung and ribbed to raised.
Strengthening industry collaboration in Hong Kong
Hong Kong Institute of Construction and The Chartered Institute of Building sign Memorandum of Understanding.
A detailed description from the experts at Cornish Lime.
IHBC planning for growth with corporate plan development
Grow with the Institute by volunteering and CP25 consultation.
Connecting ambition and action for designers and specifiers.
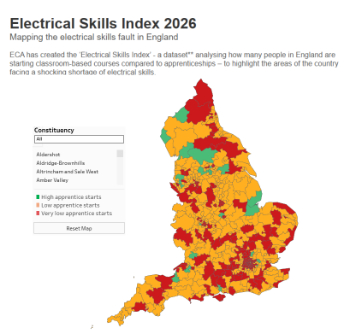
Electrical skills gap deepens as apprenticeship starts fall despite surging demand says ECA.
Built environment bodies deepen joint action on EDI
B.E.Inclusive initiative agree next phase of joint equity, diversity and inclusion (EDI) action plan.
Recognising culture as key to sustainable economic growth
Creative UK Provocation paper: Culture as Growth Infrastructure.