Primary non-rechargeable batteries
Contents |
[edit] Introduction
A primary battery is a non-rechargeable, single-use battery as opposed to a secondary cell that can be recharged. A primary battery converts chemical energy into electrical energy by means of an electrochemical process. The electrochemical process that occurs is spontaneous Oxidation-Reduction (redox) this is where both a reduction reaction and an oxidation reaction take place at once. The earliest electrochemical cells were called wet cells as electrodes were submerged in a solution, later and more modern primary cells are more commonly dry cells.
A secondary or rechargeable battery acts as a galvanic cell when it is discharging, as it is converting chemical energy to electrical energy in a redox reaction but acts as an electrolytic cell when it is being charged as it is converting electrical energy to chemical energy. Electrolysis is the process by which ionic substances are broken down into simpler substances when an electric current is passed through them, thus recharging the battery's electrical potential.
In a redox reaction, electrons accumulate on the oxidation electrode (anode) and provide a negative potential, whilst a reduction process occurs at the reduction electrode (cathode), where a positive potential develops. The two electrodes are connected (called a salt bridge), so electrons flow from the oxidation electrode to the reduction electrode in an outer circuit due to the difference in potential between them, this produces an electric current.
[edit] Development
This type of primary cell where chemical energy produced in a redox reaction is converted to electrical energy is called either a galvanic or a voltaic cell and normally a wet cell. It is so-called firstly after the scientist Luigi Galvani (1737 – 1798) the pioneer of bioelectromagnetics who discovered that a frog's leg contracts when two different metals in contact touch different parts of the muscle, he initially named this animal electricity. Alessandro Volta later created the same effect using non-biological materials to challenge the animal electricity theory with his own metal-metal contact electricity theory, he is attributed as the inventor of the first electrical battery and this approach formed the basis of the modern-day primary battery that is still in use today.
In 1836 John Frederic Daniell, a British chemist and meteorologist, invented an improved primary cell called the Daniell cell. It consisted of an earthenware container filled with sulfuric acid and a zinc electrode immersed into a copper pot filled with a copper sulfate solution. This cell, which improved on other cells was itself improved on by the gravity cell invented in the 1860s by Frenchman Callaud.
The general-purpose primary zinc-carbon cell as is commonly used today stemmed from what was known as known as the Leclanché or dry cell, after the French engineer Georges Leclanché who invented it in 1866. This battery comprised a zinc alloy sheet containing small amounts of lead, cadmium, and mercury, a saturated aqueous solution of ammonium chloride, and impure manganese dioxide blended with carbon black and electrolyte formed around an electrode. It remains the basic design of the zinc-carbon dry cells available today with variations such as the zinc chloride battery.
Alkaline as opposed acid-based batteries were first developed by Waldemar Jungner in 1899, and, also separately by Thomas Edison in 1901. Showing greater safety and power potential, the Canadian engineer Lewis Urry invented the modern alkaline dry battery in the 1950s, which uses a zinc manganese dioxide.
[edit] Current use
Today alkaline batteries are the most common type of batteries in the world because of their stable, long-lasting, mobile performance, but zinc-carbon batteries are also still used as they remain cheaper. These come in various sizes with various voltages but AAA, AA, A-C batteries for example can be found in many small household appliances. Button/coin or watch cells often use the same components but are small enough to fit in smaller devices.
[edit] Related articles on Designing Buildings
Featured articles and news
A case study and a warning to would-be developers
Creating four dwellings for people to come home to... after half a century of doing this job, why, oh why, is it so difficult?
Reform of the fire engineering profession
Fire Engineers Advisory Panel: Authoritative Statement, reactions and next steps.
Restoration and renewal of the Palace of Westminster
A complex project of cultural significance from full decant to EMI, opportunities and a potential a way forward.
Apprenticeships and the responsibility we share
Perspectives from the CIOB President as National Apprentice Week comes to a close.
The first line of defence against rain, wind and snow.
Building Safety recap January, 2026
What we missed at the end of last year, and at the start of this...
National Apprenticeship Week 2026, 9-15 Feb
Shining a light on the positive impacts for businesses, their apprentices and the wider economy alike.
Applications and benefits of acoustic flooring
From commercial to retail.
From solid to sprung and ribbed to raised.
Strengthening industry collaboration in Hong Kong
Hong Kong Institute of Construction and The Chartered Institute of Building sign Memorandum of Understanding.
A detailed description from the experts at Cornish Lime.
IHBC planning for growth with corporate plan development
Grow with the Institute by volunteering and CP25 consultation.
Connecting ambition and action for designers and specifiers.
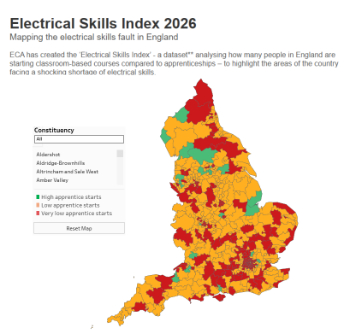
Electrical skills gap deepens as apprenticeship starts fall despite surging demand says ECA.
Built environment bodies deepen joint action on EDI
B.E.Inclusive initiative agree next phase of joint equity, diversity and inclusion (EDI) action plan.
Recognising culture as key to sustainable economic growth
Creative UK Provocation paper: Culture as Growth Infrastructure.
Futurebuild and UK Construction Week London Unite
Creating the UK’s Built Environment Super Event and over 25 other key partnerships.
Welsh and Scottish 2026 elections
Manifestos for the built environment for upcoming same May day elections.
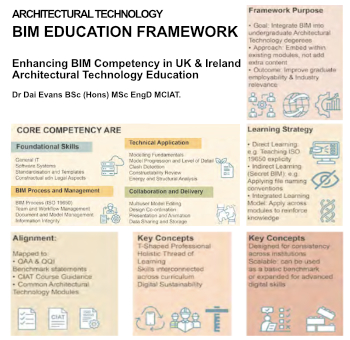
Advancing BIM education with a competency framework
“We don’t need people who can just draw in 3D. We need people who can think in data.”