Electrochemistry
Electrochemistry is a branch of chemistry concerned with electric currents in relation to chemical reactions. Certain spontaneous chemical reactions can generate useful electric currents, whilst other chemical reactions can be forced to proceed by an electric current. Electrochemistry is the basic science behind standard batteries which are also called electrochemical cells.
Electrochemistry is also the basis of many everyday household chemical products. Bleach is made from chlorine and caustic soda, which are the products of brine electrolysis and can be made directly with an electrochemical cell. Chlorine is used to treat pools as well as drinking water, and is also an ingredient in PVC. Many cleaning agents, detergents, soaps and even paper are made or treated with the caustic soda, which is a product of brine-electrolysis.
Electrochemistry is also used to make aluminium, as it is the only economically practical way to produce the metal from its ore. Other common metals such as copper, zinc, silver and lead, are refined or purified by electrochemical processes. Many of these metals may need protection from unwanted corrosion, and this can be achieved by applying a corrosion resistant metal coating or in the case of anodising an integrated substrate. In most cases this is carried out by a process called electroplating, such as with chrome, gold or silver plating, Electroforming is where whole items are created by an electrodeposition process.
Rust in metals is the result an anodic reaction which itself is the mechanism of electrochemical corrosion, where the metal forming the anode dissolves in the electrolyte in the form of positively charged ions. There can be around 6 different types of electrochemical reactions that occur when metals become corroded, so electrochemistry is related to both the problem and the solution.
[edit] Related articles on Designing Buildings
- Brass.
- Brittle fracture.
- Corrosion coupons.
- Corrosion inhibitor.
- Corrosion resistance.
- Corrosion resistant alloy CRA.
- Crevice corrosion.
- Deterioration.
- Failure of cast iron beams.
- Galvanised steel.
- Galvanic corrosion.
- Graphitisation.
- Guidance for construction quality management professionals: Structural Steelwork.
- Hydrogen embrittlement.
- Iron.
- Marine corrosion.
- Microbiologically influenced corrosion.
- Pitting.
- Rust.
- Steel.
- Stainless steel.
- Types of metal.
- Types of steel.
- Under-deposit corrosion.
Featured articles and news
A case study and a warning to would-be developers
Creating four dwellings... after half a century of doing this job, why, oh why, is it so difficult?
Reform of the fire engineering profession
Fire Engineers Advisory Panel: Authoritative Statement, reactions and next steps.
Restoration and renewal of the Palace of Westminster
A complex project of cultural significance from full decant to EMI, opportunities and a potential a way forward.
Apprenticeships and the responsibility we share
Perspectives from the CIOB President as National Apprentice Week comes to a close.
The first line of defence against rain, wind and snow.
Building Safety recap January, 2026
What we missed at the end of last year, and at the start of this...
National Apprenticeship Week 2026, 9-15 Feb
Shining a light on the positive impacts for businesses, their apprentices and the wider economy alike.
Applications and benefits of acoustic flooring
From commercial to retail.
From solid to sprung and ribbed to raised.
Strengthening industry collaboration in Hong Kong
Hong Kong Institute of Construction and The Chartered Institute of Building sign Memorandum of Understanding.
A detailed description from the experts at Cornish Lime.
IHBC planning for growth with corporate plan development
Grow with the Institute by volunteering and CP25 consultation.
Connecting ambition and action for designers and specifiers.
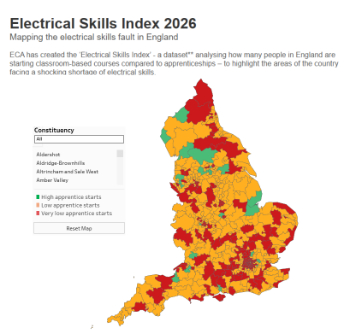
Electrical skills gap deepens as apprenticeship starts fall despite surging demand says ECA.
Built environment bodies deepen joint action on EDI
B.E.Inclusive initiative agree next phase of joint equity, diversity and inclusion (EDI) action plan.
Recognising culture as key to sustainable economic growth
Creative UK Provocation paper: Culture as Growth Infrastructure.