Specific heat capacity
The term ‘specific heat’ (or specific heat capacity) refers to the heat energy per unit mass (typically 1 kg) required to raise the temperature of a substance by one degree Celsius.
The formula for specific heat: q=mc(Tf-Ti)
The higher the specific heat capacity of a substance, the more energy is required to raise its temperature.
Specific heat capacity (c) in J (joules) / kg °C can be calculated as:
c = E/m θ
Where:
- E is the energy transfer in J.
- m is the mass of the substances in kg.
- θ is the temperature change in °C.
Some examples of the specific heat capacities of different substances are listed below:
- Aluminum 902 J/kg°C
- Copper 385 J/kg°C
- Gold 129 J/kg°C
- Iron 450 J/kg°C
- Lead 128 J/kg°C
- NaCl 864 J/kg°C
- Oxygen 918 J/kg°C
- Water 4181 J/kg°C
- Brick / block: 840 J/kg°C
- Concrete: 880 J/kg°C
- Marble: 880 J/kg°C
- Steel: 420 J/kg°C
- Timber: 1200 J/kg°C
Specific heat capacity is one of the properties that contributes to the thermal mass of a material, that is, how much heat it can store. Water, which has a very high specific heat capacity, is very effective at storing heat.
[edit] Related articles on Designing Buildings Wiki
Featured articles and news
National Apprenticeship Week 2026, 9-15 Feb
Shining a light on the positive impacts for businesses their apprentices and the wider economy alike.
Applications and benefits of acoustic flooring
From commercial to retail.
From solid to sprung and ribbed to raised.
Strengthening industry collaboration in Hong Kong
Hong Kong Institute of Construction and The Chartered Institute of Building sign Memorandum of Understanding.
A detailed description fron the experts at Cornish Lime.
IHBC planning for growth with corporate plan development
Grow with the Institute by volunteering and CP25 consultation.
Connecting ambition and action for designers and specifiers.
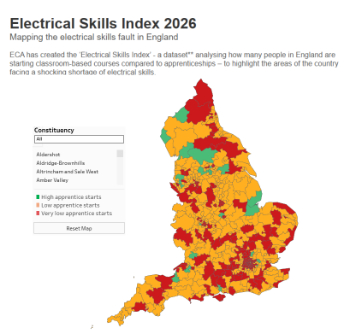
Electrical skills gap deepens as apprenticeship starts fall despite surging demand says ECA.
Built environment bodies deepen joint action on EDI
B.E.Inclusive initiative agree next phase of joint equity, diversity and inclusion (EDI) action plan.
Recognising culture as key to sustainable economic growth
Creative UK Provocation paper: Culture as Growth Infrastructure.
Futurebuild and UK Construction Week London Unite
Creating the UK’s Built Environment Super Event and over 25 other key partnerships.
Welsh and Scottish 2026 elections
Manifestos for the built environment for upcoming same May day elections.
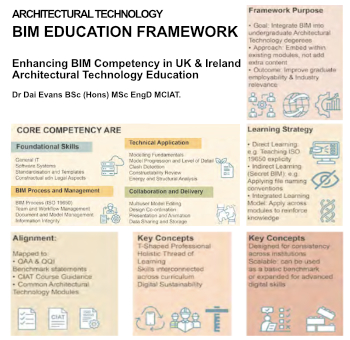
Advancing BIM education with a competency framework
“We don’t need people who can just draw in 3D. We need people who can think in data.”
Guidance notes to prepare for April ERA changes
From the Electrical Contractors' Association Employee Relations team.
Significant changes to be seen from the new ERA in 2026 and 2027, starting on 6 April 2026.
First aid in the modern workplace with St John Ambulance.
Solar panels, pitched roofs and risk of fire spread
60% increase in solar panel fires prompts tests and installation warnings.
Modernising heat networks with Heat interface unit
Why HIUs hold the key to efficiency upgrades.